Surprising antibiotic discovery could pave way for new family of high blood pressure treatments
Scientists from the UK and South Africa have discovered a surprising new role for a commonly-used antibiotic that could open the door to a new family of improved therapies for high blood pressure and cardiovascular disease.
The international team, led by Professor Ravi Acharya from the University of Bath (UK) and Professor Ed Sturrock from the Institute of Infectious Disease and Molecular Medicine (IDM), University of Cape Town (South Africa), have found that the antibiotic ciprofloxacin blocks an enzyme involved in regulating blood pressure, called ACE (Angiotensin-Converting Enzyme).
Importantly, they have identified it blocks ACE in a different way to other ACE inhibitors, offering a novel perspective on how to design improved pharmaceuticals to tackle cardiovascular diseases with potentially fewer side effects. Around one in three adults in the UK suffer from high blood pressure, with around 12 million people taking ACE inhibitor medications to treat it. ACE increases blood pressure by converting an inactive molecule called angiotensin I into angiotensin II, which narrows (or constricts) blood vessels. ACE inhibitor medications block this process thereby reducing blood pressure. However ACE is also involved in other chemical reactions that affect a range of physiological functions including kidney function, reproduction and the immune response. As a result of this “promiscuity”, current medications that block ACE affect multiple bodily processes, causing unwanted side effects such as coughing or swelling of the throat and tongue.
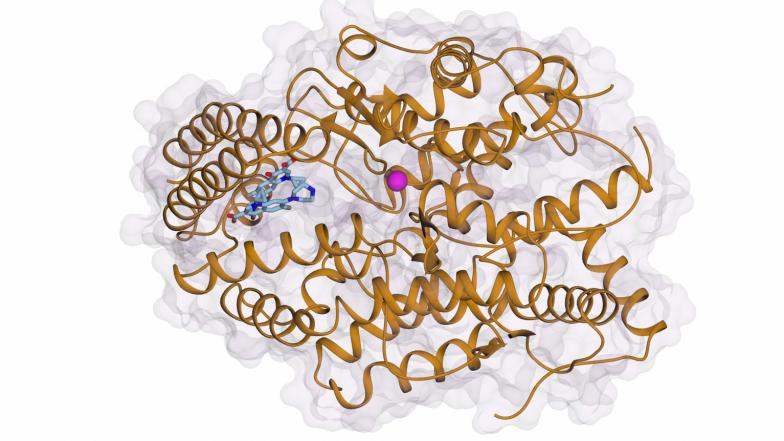
The ACE enzyme has two parts, termed the N-domain and C-domain. The two parts harbour a pocket, or active site. Current inhibitors bind to this site, filling the pocket and stopping the enzyme from working. In their study, published in ACS Bio & Med Chem Au, the authors show that ciprofloxacin binds selectively to a different “allosteric” site in the C-domain, which blocks angiotensin I from binding but does not inhibit the enzyme’s other functions.
While ciprofloxacin binds too weakly to be effective as a treatment itself, the team suggests it could be used as a template for a new family of drugs based on the same chemical structure, with the aim of developing better, more targeted ACE inhibitors that could have fewer side effects.
Professor Ravi Acharya, from the University of Bath’s Department of Life Sciences, said: “This groundbreaking research not only advances our understanding of ACE regulation but also highlights the potential for creating next-generation inhibitors that are safer and more efficient in managing hypertension and cardiovascular disorders. Our study brings a fresh twist by identifying the antibiotic ciprofloxacin as an allosteric inhibitor of ACE - instead of latching onto the active site like traditional inhibitors, ciprofloxacin cleverly binds to an exosite within the C-domain, away from the catalytic pocket.”
Funded by the Biotechnology and Biological Sciences Research Council (UKRI-BBSRC), the research brought together a team of biochemists from Acharya’s and Sturrock’s groups who have been collaborating for 30 years. Dr Vinasha Ramasamy and Professor Ed Sturrock from the University of Cape Town investigated the enzyme reaction kinetics of ACE, whilst Dr Kyle Gregory and Professor Ravi Acharya from the University of Bath determined the 3D molecular structure of ACE using X-ray crystallography techniques.
The team plans next to screen different chemical analogues of ciprofloxacin to optimise binding and specificity of this new family of inhibitors.
Read the full article here.
